Summary
- Nanoform and TargTex collaborated on an innovative hydrogel-based pharmaceutical product for the treatment of glioblastoma after surgery. Glioblastoma is the most common type of malignant primary brain tumor that is difficult to treat and has a high risk of recurrence after surgery.
- The nanoformed API demonstrated superior properties, enabling a fivefold increase in drug load compared to that obtained by nanomilling, the less effective alternative.
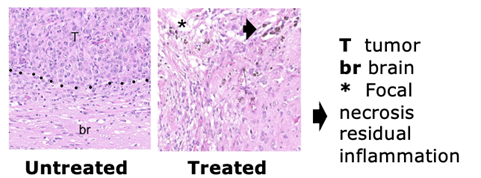
- In vivo data and imaging did not detect a tumor with the treatment at a dose under the maximum loading dose in rats.
- This animal data supports the progression of this program to human clinical studies. Furthermore, Nanoform will investigate the feasibility of nanoforming other APIs for use with implantable gels and devices
Background
Glioblastoma multiforme (GBM) is the most common type of malignant primary brain tumor, with patients facing, in most cases, a life expectancy of less than 16 months. There is a need for efficacious treatments for this aggressive brain cancer.
Challenges
TargTex, a European biotech company, is developing an innovative hydrogel-based pharmaceutical product to target GBM. The hydrogel is implanted peri-operatively, following which it diffuses across the brain parenchyma. The first prototype hydrogel was effective for primary tumor eradication, but did not stop tumor recurrence. The original formulation also had limited dosing possibilities. In order to optimize the hydrogel, TargTex set the following parameters for its drug candidate to succeed:
- Increased drug loading
- Controlled drug release
- Sterilizable, non-toxic, biocompatible, biodegradable
- Thermo-responsiveness
- Utilizing excipients approved by regulatory bodies
TargTex is collaborating with Nanoform to enhance its GBM hydrogel and improve its diffusion across the brain parenchyma. This follows a successful PoC (Proof of Concept) study. In this PoC project, the hydrogel formulation, developed by Nanoform and as a result of nanoparticles created using Nanoform’s CESS® nanoparticle technology, enabled a fivefold increase in drug load compared to nanomilling – the less effective alternative.
Results
TargTex’s drug candidate in the Nanoform hydrogel formulation has already shown efficacy in animal models, with imaging modalities failing to detect tumors in all rodents at a dose below the maximum loading dose. Out of the total rodents in the study, 40% experienced long term survival and no evidence of tumor recurrence or residual cancer cells present after sacrifice.
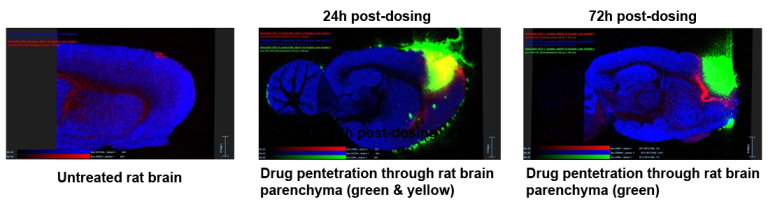
Successfully applying Nanoform’s proprietary CESS® technology, deep brain penetration by the drug API has been demonstrated by mass spectrometry imaging).
Pharmacokinetic data show no systemic exposure. The drug candidate is non-toxic at maximum loading concentration.
Future work
Having developed a GMP-scalable formulation, Nanoform will continue to scale up and optimize the manufacture of this promising GBM therapeutic with the aim of increasing the drug load and improving the long-term survival rate.
The next step for the project is for TargTex to progress along a path to clinical studies. The two companies also hope to continue to study the benefits nanoforming can bring to other novel oncology medicines for patients in need.