
Small is Powerful for small and large molecules
Nanoform’s Controlled Expansion of Supercritical Solutions (CESS®) technology is uniquely capable of producing pure API particles as small as 10 nm.
- A novel processing platform that delivers unique nanoparticles which:
- Enable high drug-load formulations e.g fewer, smaller tablets than possible with alternative technologies
Enhances bioavailability and increases probability of success in clinical development.

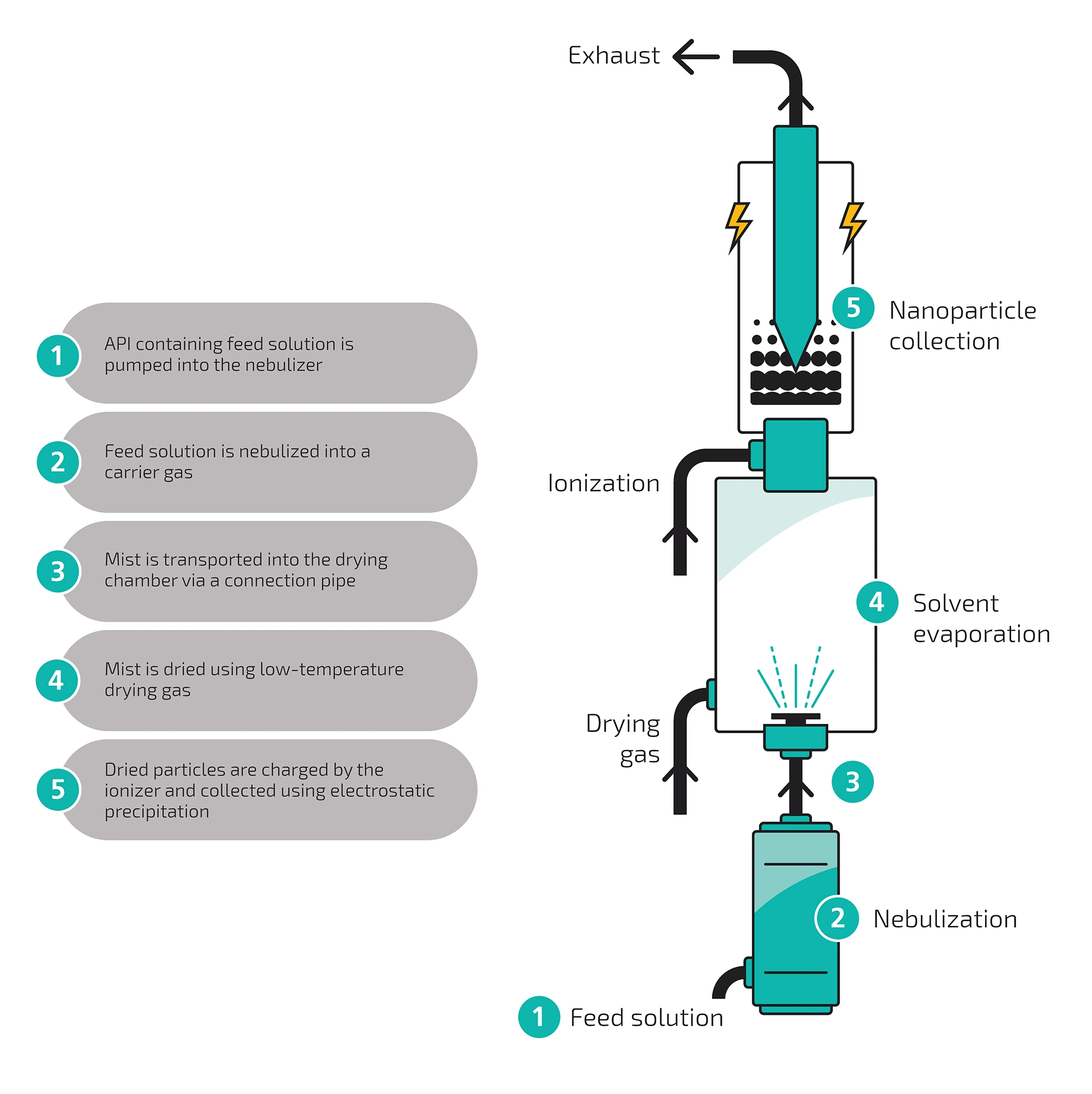
- Recycled pharma grade CO2 is supplied at scale to the CESS® lines
- API is loaded, CO2 is introduced, with pressure and temperature increased to create supercritical state (scCO2), which dissolves the API
- The scCO2-based API solution flows through the pressure lines
- The pressure is reduced under specific parameters, to control nucleation; forming nanoparticles
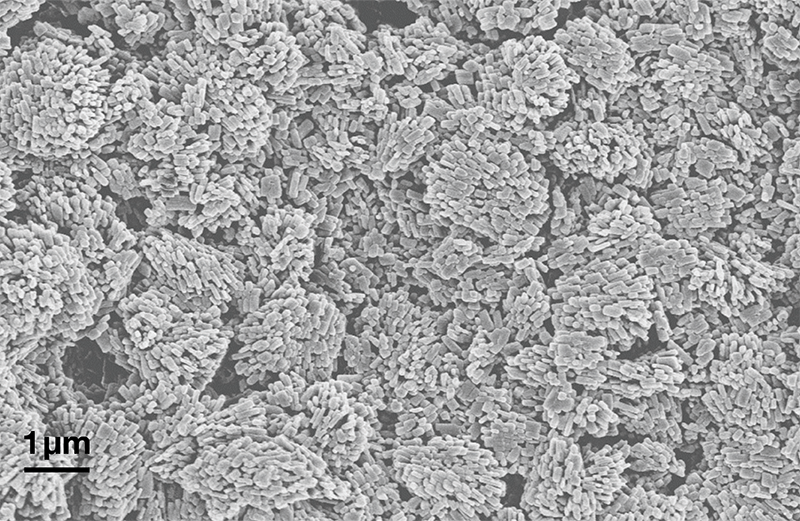
- Pressure is further reduced during atomization, rapidly expanding CO2 freezes and dry ice is collected in which API nanoparticles are embedded. After the sublimation of dry ice, nanoformed API is obtained. SEM image of nanoformed API.
Nanoform’s CESS® technology is designed to overcome solubility and bioavailability challenges in drug development. The process produces nanoparticles as small as 10 nanometers without reliance on excipients, inorganic carriers or complex formulation. The technology uses a green solvent, supercritical carbon dioxide (scCO₂). The API dissolves in scCO₂ under precisely controlled temperature and pressure conditions. The solution undergoes a controlled expansion where the solubilization capacity of CO₂ rapidly decreases, forming nanoparticles. The process is tightly controlled to yield highly uniform nanoparticles with tunable distribution of size and morphology. The resulting particles are suitable for downstream processing into various drug delivery systems such as oral tablets, inhalable powders, or injectable formulations.
Advantages of Nanoforming:
- Higher dissolution rate, apparent solubility and bioavailability: by reducing particle size and increasing surface area through nanoparticles, molecules can be progressed into clinical development without the need for amorphous solid dispersion (ASD) technology, and at the same time enable fewer, smaller tablets, reducing pill burden, and potentially improving patient compliance
- Without reliance on solvents, excipients, or surfactants, nanoparticle formulation is simplified
- Scalable, recyclable, and sustainable: the process is environmentally friendly, using carbon dioxide as a green solvent, eliminating the need for organic solvents and we are looking into recycling the CO2 used to become carbon negative.
Applications (from 1g to 10 tonnes)
- High drug load, high bioavailability oral dosage forms: fewer, smaller tablets
- Enabling alternative delivery routes: CESS® formed nanoparticles can also be utilized for:
- High drug load injectables and improved ophthalmic formulations
- High drug load nanosuspension for nebulization or dry powder inhalation nanoformulations for low soluble API.
Read more about fine tuning the polymorphic form of CESS® nanoparticles
Publications to be downloaded:
- Pessi et al, J Pharm Sci, 2016, DOI: 10.1016/j.xphs.2016.05.022
- Kauppinen et al, J Pharm Biomed Anal, 2023, DOI: 10.1016/j.jpba.2022.115169
Nanoform Biologics platform
Nanoform’s proprietary Biologics nanoparticle engineering platform can produce pure API or API-excipient combination nanoparticles as small as 50 nm.
The patented Nanoform Biologics platform allows the manufacturing of dry particles in a low temperature, low shear force environment. The process employs nebulization followed by ambient drying to give precise control over the size and morphology of particles, and the possibility of simple coprocessing with excipients and carriers.
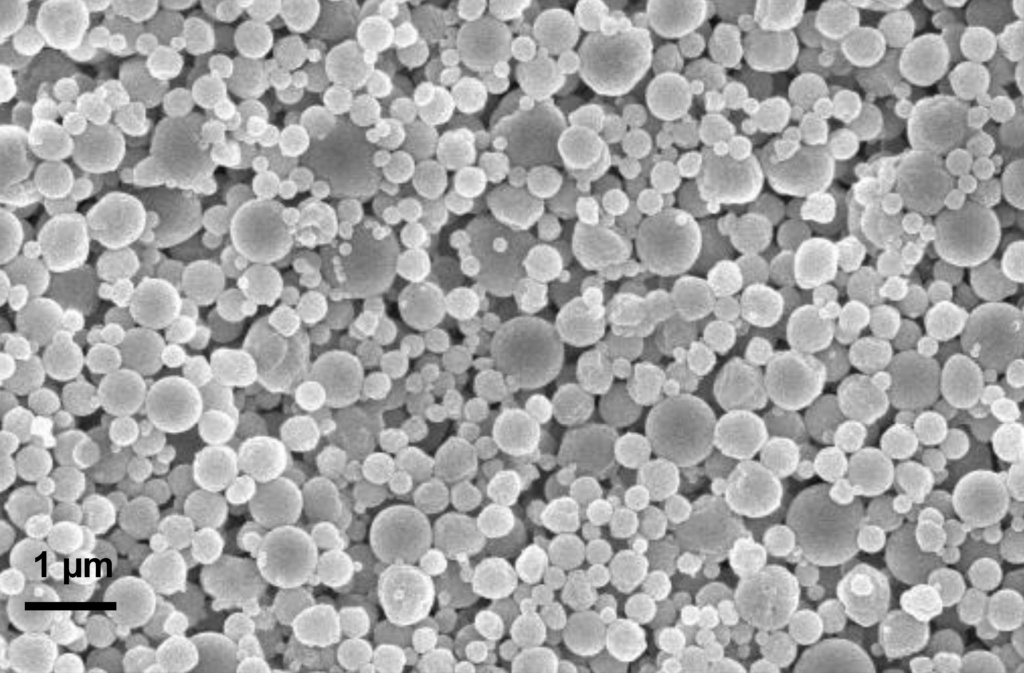
The image above shows the typical output from our Nanoform Biologics platform, with mean particle size of 250 nm. Currently, we have five R&D lines available for preclinical development projects. A scaled-up, GMP-like line is also ready to tackle development challenges. We are planning sterile GMP manufacturing capabilities to meet our customers clinical development needs.
Would you like to learn more about our plans for GMP manufacturing? Please contact us here and we would be delighted to discuss how we plan to take your molecule into clinic and beyond.