Our technologies
Small molecules
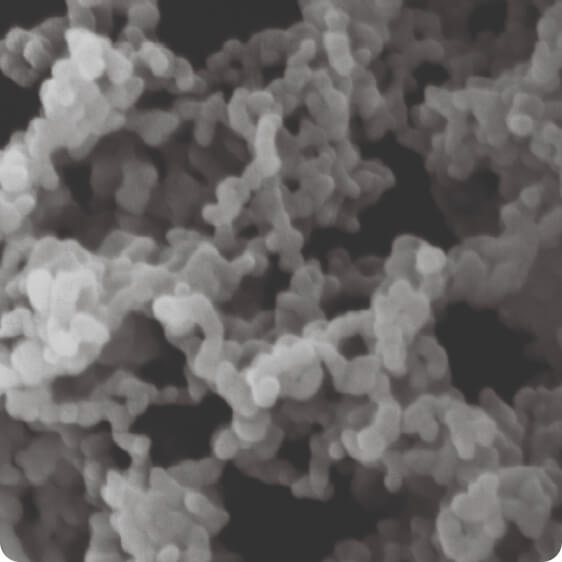
Our multi-patented Controlled Expansion of Supercritical Solutions (CESS®) technology enables the creation of API nanoparticles directly from solution. The process can be applied to most small molecules, with a high success rate.
Biologics
Our biological nanoforming technology can deliver large-molecule drug particles as small as 50 nm while retaining biological activity. It’s effectiveness has been demonstrated on proteins in the 1-150 kDa range, and it is capable of engineering particle sizes to specific requirements.
Formulation
Our experienced formulation development team designs and develops flexible dosage forms of nanoformed drug substances to meet preclinical and clinical development as well as life-cycle needs. Our dosage forms cover oral, parenteral, ophthalmic and inhaled therapies and more.
GMP Manufacturing
We have GMP manufacturing capabilities for the manufacture of nanoformed clinical-grade API’s (Active Pharmaceutical Ingredients) for our customers and partners.
HIGH POTENCY CAPABILITIES
LATEST PRESS RELEASES & NEWS
To read more of our latest news and see our recent articles and videos, visit our media centre