
how small is small?
Analytical chemistry plays a crucial role in characterising and understanding materials made from nanoforming and formulation processes. We use a variety of techniques to analyze our nanoparticles and formulations and ensure that they meet strict quality and safety standards. Our analytical team utilizes state-of-the-art equipment and software to accurately measure the properties of our nanoparticles, including purity, size, shape and crystallinity. This information is essential for understanding how to develop our formulations and predict how our drugs will interact in-vivo so as to optimize their efficacy.
The physical solid-state properties of our nanoparticles are also a key area of focus for our team. By carefully manipulating these properties, we can control solubility, stability, and bioavailability.
Read
our article
Small is Powerful: A Globally Unique Capability for Nanoforming HPAPIs

Our main capabilities
Nanoparticle characterization is a critical aspect of our pharmaceutical development, as it allows us to understand the physical and chemical properties of both the active pharmaceutical ingredient (API) nanoparticles and the formulated nanoparticles. Here are some of the key pieces of equipment that we use for nanoparticle characterization.
- Dynamic Light Scattering (DLS): Malvern Zetasizer Ultra DLS ZSU5700
- Scanning Electron Microscopy (SEM): Coxem EM-30AX , Zeiss Gemini and Hitachi Regulus SU8220 FE-SEM
- X-Ray Powder Diffraction (XRPD): Malvern PanAnalytical Empyrean Series 3
- Thermogravimetric Analysis (TGA): TA TGA550
- Ultra-Performance Liquid Chromatography (UPLC) and High-Performance Liquid Chromatography (HPLC): Waters Acquity H UPLC Plus, Acquity Arc, Agilent 1290 Infinity II UHPLC, Waters Acquity Premier (Bio), Arc Premier (Bio)
- Atomic Force Microscopy (AFM): Park Systems NX10
- Differential Scanning Calorimetry (DSC): TA DSC Q2000 and TA DSC 250
Analytical Development and Method Validation
Analytical method development and validation are critical aspects of our drug development process. It involves establishing that our analytical methods are reliable, accurate, and reproducible. There are several parameters that need to be met during method validation, including specificity, accuracy, precision, linearity, range, and limit of detection and quantification. The specific requirements for each parameter can vary depending on the analytical technique being used and the type of sample being analyzed.
At Nanoform we are committed to the QbD approach to pharmaceutical development. We use state-of-the-art analytical methods, statistical and modelling tools, and risk-based approaches to design and develop drug products that meet the needs of individual patients, while adhering to strict regulatory standards.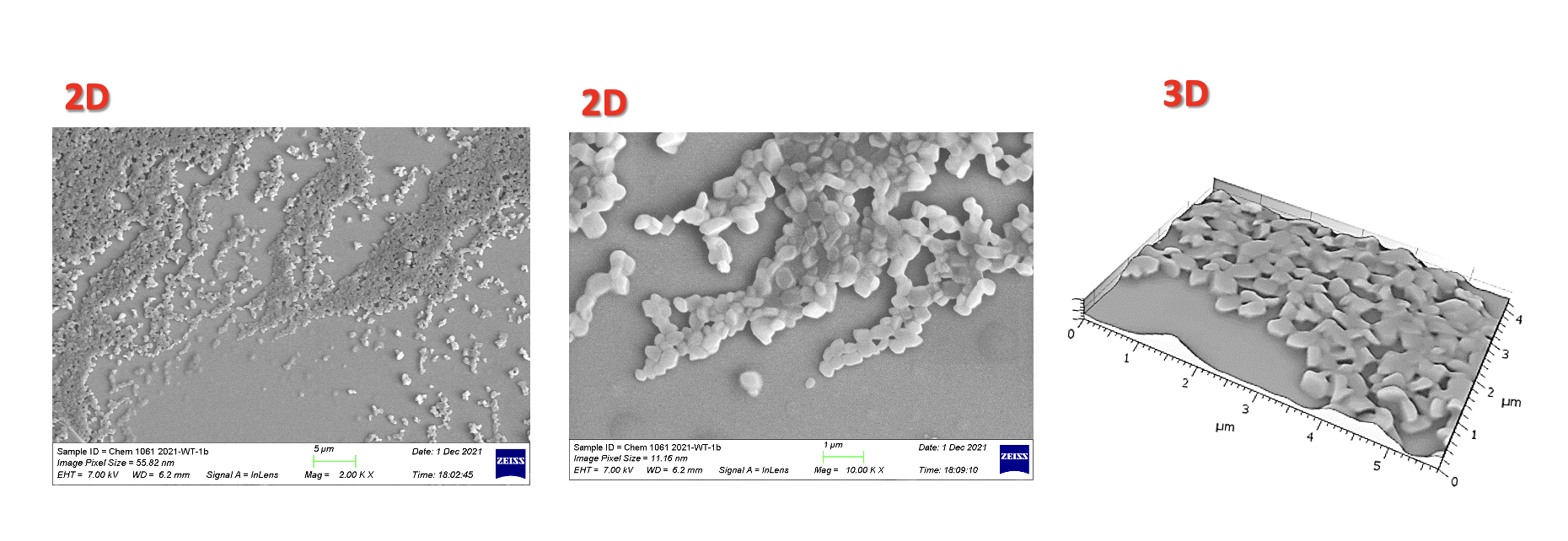
Nanomicroscopy of Monolayered API Nanoparticles – SEM 2D and 3D features
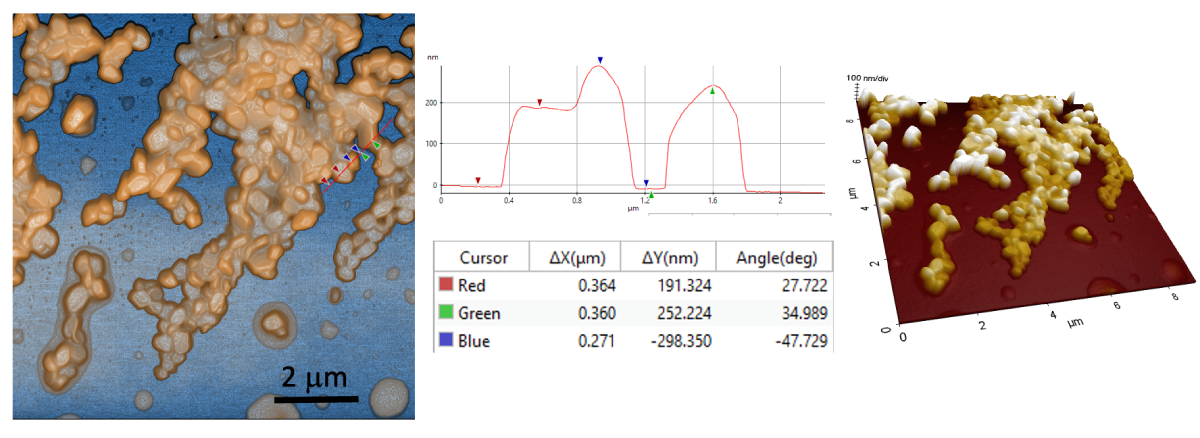
Nanomicroscopy of Monolayered API Nanoparticles – AFM 3D features
Get in touch to find out how our CESS® technology can help your small-molecule drugs reach their full therapeutic potential