Background
This study sought to clarify the effect of drug particle size on drug release kinetics from highly-loaded ethylene vinyl acetate (EVA) implants. In highly-loaded monolithic implants, drug release occurs through a network of pores created by dissolved drugs. Changing particle size alters the polymer – drug physical interactions and was expected to impact drug release kinetics.
Challenges
EVA implants are well-established for long-acting subcutaneous drug delivery, with multiple approved drug products. It is desirable to produce implants with as high of a drug loading as possible, but there are associated challenges – at high loadings, monolithic implants sometimes exhibit a high initial burst release and greater than desired overall release rate. The standard solution used by multiple marketed products is to produce multilayer implants consisting of a highly loaded core with a rate-limiting outer layer– this layer reduces the burst release and can be used to tune drug release kinetics in a manner that is independent of drug loading in the core.

There are cases where designing a multilayer implant is inconvenient, however, so there is a drive to develop alternative methods to optimize drug release kinetics. To solve this challenge, Nanoform and Celanese, a global specialty chemicals company, collaborated to assess the utility of combining Nanoform’s Controlled Expansion of Supercritical Solutions (CESS®) nanoforming technology with Celanese’s VitalDose® EVA copolymer delivery technology for drug-eluting implants.
Results
CESS® produced nanoparticles of two potent drugs, risperidone (an atypical antipsychotic) and fingolimod (an immunomodulating compound) resulted in a slower release rate in EVA implants relative to micronized material.
Nanoformed risperidone also exhibited a much lower initial burst release than micronized risperidone, which was tested as the control.
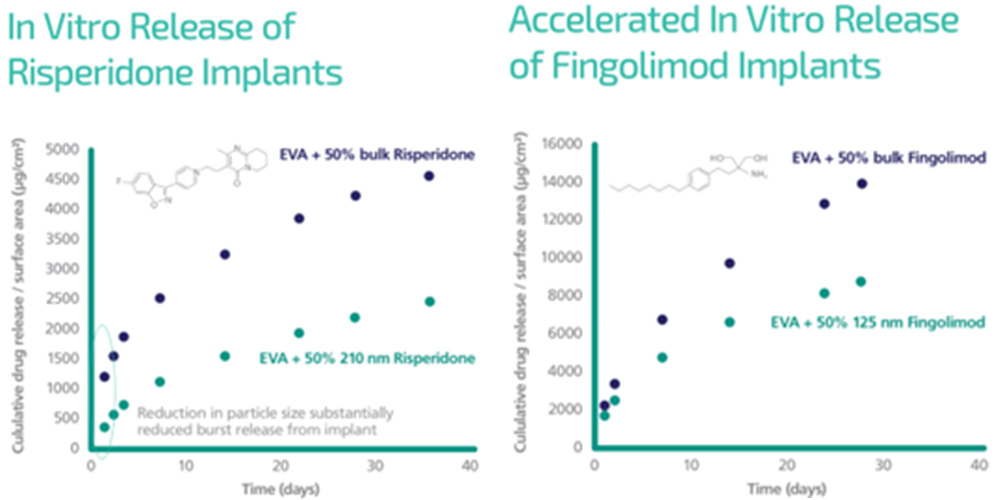
These results demonstrate the exciting potential of CESS® nanoparticle engineering as an alternative method to solve the long-standing challenge associated with the drug release kinetics of implants for long-acting subcutaneous delivery.