Summary: Nanoform Biologics’ high-dose subcutaneous delivery platform represents a significant advancement in the field of biologics. By addressing the challenges associated with subcutaneous delivery, such as volume limitations and protein aggregation, Nanoform’s technology enables the development of highly concentrated, stable, and subcutaneously injectable biologic formulations. This innovation has the potential to revolutionize the administration of biologics, making treatments more accessible, and improving patient adherence and therapeutic outcomes. As demand for biologics continues to rise, Nanoform’s technology can play a crucial role in meeting the needs of patients and healthcare providers alike.
The global market for biologics – drugs or vaccines made from a living organism and including monoclonal antibodies (mAbs), recombinant proteins, antisense and RNAi therapeutics – was worth over $460 billion in 2022, and it is expected to achieve a compound annual growth rate of over 10% from 2023 to 2030.[1] Biologic therapies include treatments for serious diseases including cancer, neurologic disorders, metabolic/endocrine disorders, autoimmune diseases, pulmonary conditions, and ophthalmic disorders. These treatments are well tolerated and targeted and can significantly improve patient outcomes. However, their administration presents challenges due to aspects of their intrinsic nature, such as their high molecular weight, physical and chemical instability, and susceptibility to enzymatic degradation.
Historically, most biologics were developed for intravenous (IV) administration, as it is the quickest way to achieve target drug levels in the blood. The IV method typically requires that healthcare practitioners are organized to administer the required dose, imposing additional costs on the healthcare system and treatment burden for patients. To avoid such issues, subcutaneous (SC) delivery has emerged as an attractive alternative. Subcutaneous delivery offers expanded opportunities for easier or self-administration at home – potentially using auto-injectors or pre-filled syringes – and improves the patient’s experience by reducing the need for hospital visits and assisting in increasing adherence. However, SC formulations are challenging to develop, as therapeutic doses are frequently greater than 1,000 mg and, to ensure injectability, the effective protein concentration achievable in solution is rarely beyond 120 mg/mL.
And while IV delivery can deliver large volumes of medication, SC administration is limited in volume due to restrictions of the extracellular matrix, the network of extracellular macromolecules that provide structural and biochemical support to cells surrounding the site of injection. The limitation on volume poses the additional formulation challenge of increasing concentration to facilitate SC delivery of high doses. Most biologic molecules have an exponential relationship between concentration and viscosity, affecting manufacturability, stability, and injectability of the drug product.
Current strategies rely on the use of hyaluronidase enzyme, co-administered alongside the active ingredient to degrade the cellular structure around the injection site and create a cavity for medicine deposition, thus allowing for larger volumes to be delivered by the SC route. Such approaches are limited by their requirement for on-body auto-injectors, and the associated time-in-hospital to oversee administration.
One promising approach is to use protein/mAb suspensions in non-aqueous vehicles, which potentially have lower viscosity compared to aqueous solutions at high protein concentrations. Protein suspensions consist of nano- to micron-sized particles comprising a therapeutic protein dispersed in a non-aqueous liquid. The solution-to-particle drying step is critical in the process as it is important that protein stability and activity are maintained, while particle size and morphology are controlled to ensure that the viscosity of the suspension permits its injectability.
Freeze-drying or lyophilization with subsequent micronization and spray-drying are conventional powder preparation techniques. However, the use of these technologies may lead to increased monomer content and aggregation, potentially impacting the quality and activity of the therapeutic protein or mAb.
The Nanoform Biologics Platform
In contrast, the patented Nanoform Biologics platform allows the manufacturing of dry particles without exposing proteins and antibodies to high temperatures and shear forces, which are often applied during spray drying. Instead, the process employs nebulization followed by ambient drying. Particle size and shape can be tuned, and the therapeutic protein can be easily co-processed with excipients or carriers.
Advantages of Nanoform Biologics Particles for SC Delivery of High-Dosed Suspensions
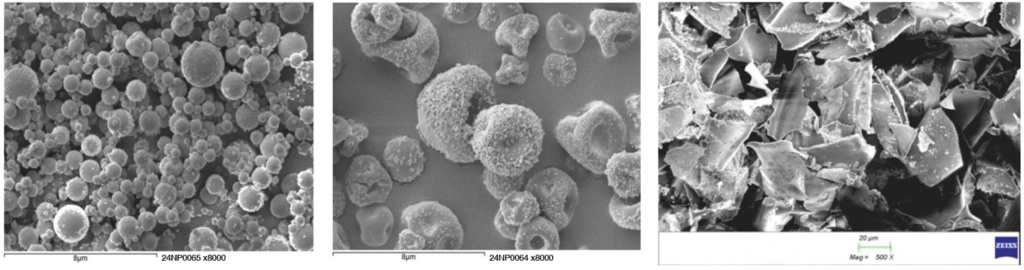
- High Concentration: Nanoform’s unique spherical particle shape and morphology enables concentrations greater than 400 mg/mL.
- Reduced Risk of Protein Aggregation: High concentration protein solutions tend to show dimerization (chemical bonding) and oligomerization (the arrangement of protein monomers into homo- or hetero-oligomers), either of which can reduce efficacy. Importantly, Nanoformed biologics maintain biological activity with no change in antibody properties, such as aggregation, size, and stability. This ensures that the therapeutic protein remains effective and safe for patient use.
- Improved Stability: Dissolved water-based products impose stability challenges. Nanoform’s gentle, bottom-up biologics platform produces nanoparticles present in formulations either as dry powders for reconstitution or suspended solid particles, minimizing contact time with water and improving stability and storage shelf life. This enhanced stability is essential for maintaining the efficacy of biologics over extended periods, contributing to a product’s shelf-life.
- Free-Flowing and Homogeneous Suspension: Using FDA-approved vehicles for the injectable route, Nanoform’s patented process ensures a free-flowing and homogeneous suspension to enable accurate dosing, which is critical to patient safety.
Case Study: Trastuzumab SC Highly Concentrated Formulation for Self-Administration
Trastuzumab, sold under the brand name Herceptin®[2] among others, is an mAb used to treat breast and stomach cancer. It is specifically used for cancer that is HER2 receptor positive. IV formulation (20 mg/mL) requires infusion times of 30 to 90 minutes, depending on dose regimen and scheduling. SC formulation is also available as a combination of trastuzumab and hyaluronidase (600 mg mAb to be reconstituted in a 5 mL solution to be injected over 2 to 5 minutes). Both require administration by a healthcare professional.
Developing a highly concentrated formulation to keep the injection volume below or equal to 2 mL would allow self-injection, and therefore greatly simplify administration set-up, conditions and patient comfort. This would also help reduce costs associated with professional healthcare. Trastuzumab “bulk” formulation is processed through the Biologics platform to enable the generation of dry particles of specific morphology and size (typically Dn50 in the 600–700 nm range with size distribution ranging from 100 to 5,000 nm). Such particles are then suspended in relevant non-aqueous solvent (in this case benzyl benzoate) achieving concentration exceeding 400 mg/mL. The maximum concentration of trastuzumab depends on the vehicle used. An injection force of below 15 N was measured when using a syringe with a standard needle gauge, and the non-Newtonian suspension viscosity was compatible with injection in standard needle gauges. This case study demonstrates the potential for Nanoform’s technology to enable the SC delivery of high-dose biologics, improving patients’ experience and treatment outcomes.
Nanoform Capabilities
The Nanoform Biologics platform offers a fully scalable process, with the ability to supply low bioburden materials for preclinical studies. The company is currently establishing GMP capabilities to supply sterile powder, adding crucial scalability for the transition from research and development to commercial production. The platform allows for the co-processing of therapeutic proteins with excipients or carriers, further enhancing the versatility and applicability of formulations. By leveraging unique particle engineering techniques, Nanoform can create highly concentrated biologic formulations that are both stable and injectable.
Conclusion
Nanoform Biologics’ innovative approach to high-dose subcutaneous delivery of biologics addresses the challenges of traditional SC delivery methods. This advancement has the potential to significantly improve patient experience and compliance, paving the way for more effective and accessible biologic therapies.
The ability to administer high doses of biologics subcutaneously offers numerous benefits, including reduced treatment burden, improved patient convenience, and increased adherence to treatment regimens. As the field of biologics continues to grow, the demand for innovative delivery methods will increase. Nanoform’s technology positions them at the forefront of this evolving landscape, offering solutions that can transform the way biologics are administered and improve patient outcomes.
References
- Grand View Research, Biologics Market Size & Trends, grandviewresearch.com/industry-analysis/biologics-market, accessed Oct. 2024
- Herceptin® (trastuzumab) is a registered trademark of Genentech