
Small is
IP Protected
Nanoforming Powers the Creation of Intellectual Property
Pharmaceutical companies can maximize their return on investment by ensuring their products have a strong intellectual property framework. Formulation development is a key enabler to improved drug product performance, innovation and problem-solving, and hence IP creation.
At its core, Nanoform’s IP is centered around its ability to manufacture unique particles, which create potential for novel formulation approaches and enable the creation of high-performance dosage forms with unique IP positions.
Nanoform has a four-layered approach to intellectual property protection:
- The first layer is the unique technology and process employed to generate nanoparticles, ensuring that the process cannot be copied
- Second, the process delivers unique particles with a high degree of control over critical characteristics
- A third layer is the formulation of nanoparticles. Besides the conventional formulation IP, we have also developed formulation platforms, e.g., in the case of small molecules, we can generate polymer embedded crystalline nanoparticles from amorphous nanoparticles post Nanoforming, for increased stability, enabling high drug load formulations whilst also increasing bioavailability
- Finally, the fourth layer provides drug product specific IP protecting the final dosage form.
Nanoform ensures its partners have a well-managed IP strategy, from the production of the nanoparticles to the finished product, with a significantly reduced risk of direct competition.
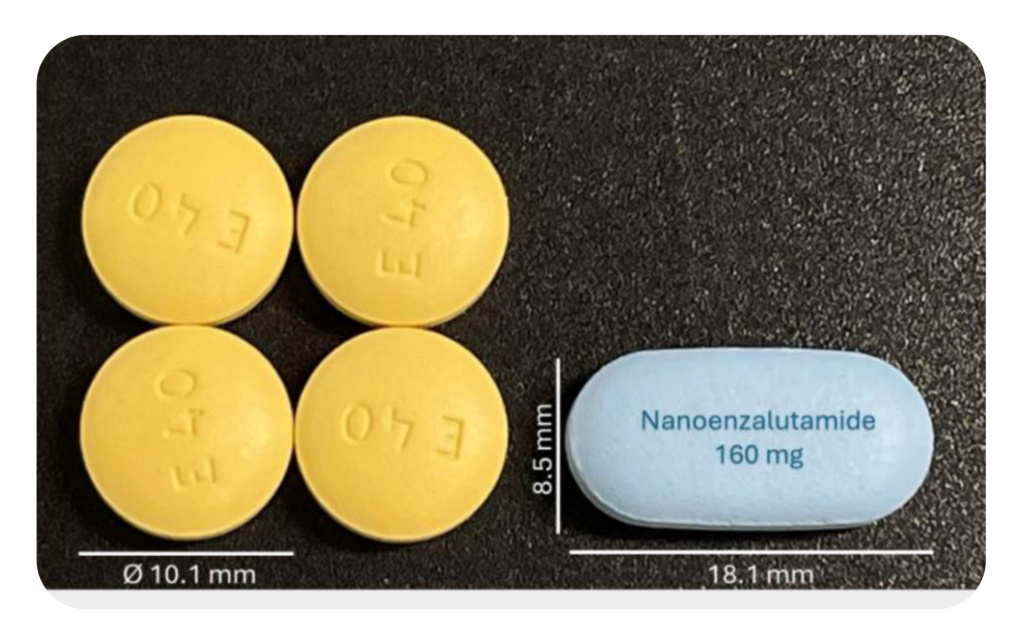
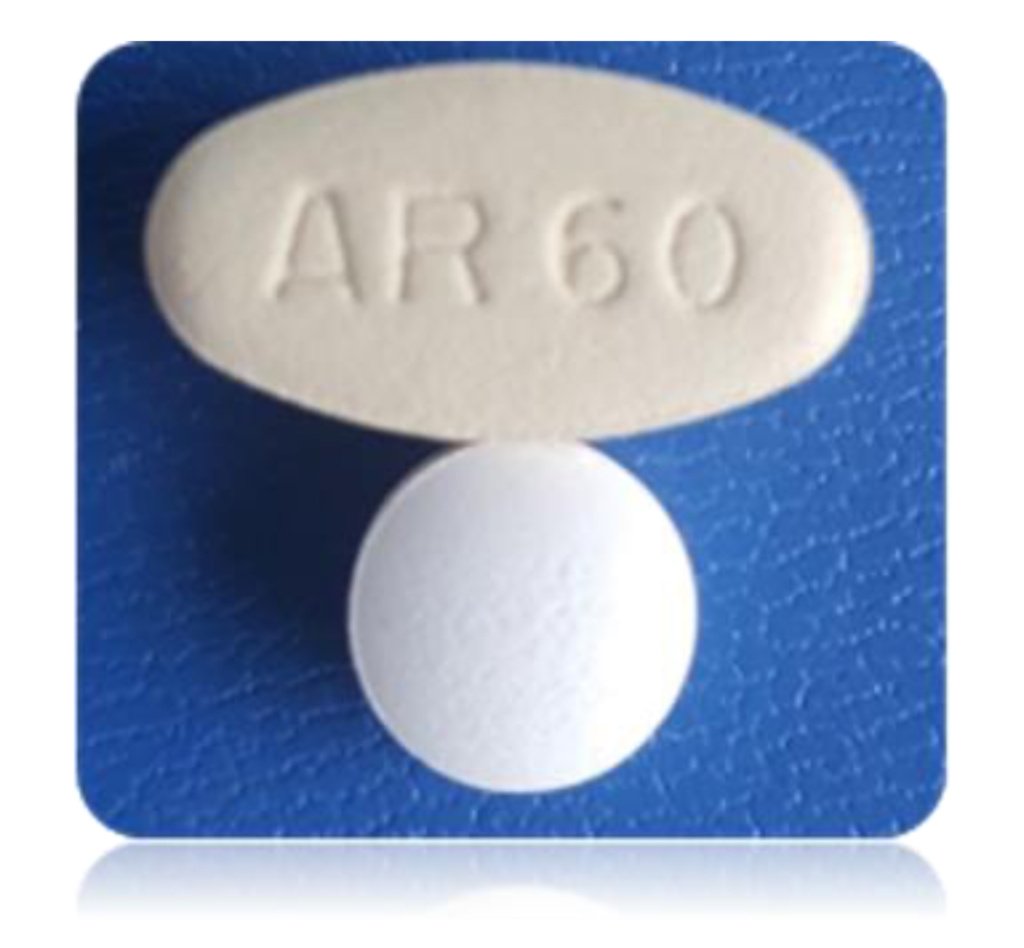
In small molecules, using Nanoform’s unique particles enable products with fewer, smaller tablets and an IP protection that restricts the ability of new entrants to manufacture an equivalent drug. Examples include Xtandi® (enzalutamide), where Nanoform technology has enabled a single tablet for delivery of an equivalent daily dosage and Erleada® (apalutamide), where nanoengineered particles have enabled a significant reduction in tablet size.
In large molecules, Nanoform is highly focused on the subcutaneous delivery of high drug-load formulations which can replace intravenous infusions of antibodies that are typically administered over a lengthy period in a clinical setting. The high drug loading, greater than 400 mg/ml of protein in non-aqueous suspensions enabled by Nanoforming opens up the potential for at-home administration with significant IP protection.
Further opportunities for IP creation exist across small and large molecules including inhalation (where there is a high level of control over fine particle fraction) and hydrogels for post-surgical implantation. Nanoform has considerable expertise in leveraging its IP to create highly tailored dosage forms with enhanced performance.
Get in touch to find out how our CESS® technology can help your small-molecule drugs reach their full therapeutic potential