
Product
Partnering
At Nanoform, our ambition is to improve the lives of one billion patients through nano-enhanced formulations. We are accelerating adoption of our technology through multiple co-development partnerships that will bring substantially improved medicines to patients around the world in the near term.
These initiatives not only deliver therapies that are more convenient and easier to administer, but also help extend the lifecycle of innovator products and create added-value generics, ensuring that patients receive the best possible treatments.
Nanoform Product Partnering overview

Read about how we enable proprietary patient centric products:
→High Drug Load Alternatives to ASDs
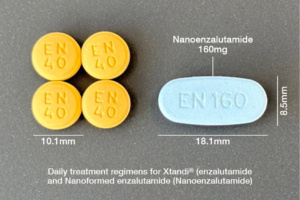
Our lead product, Nanoenzalutamide, a co-development with the On-Concept® Consortium is a patient-centric version of XTANDI® (enzalutamide), the number one prescribed androgen receptor inhibitor approved to treat prostate cancer. Planned for launch in the US and EU in 2027–2028, our nano-enhanced formulation is designed to offer a dramatically reduced pill burden for cancer patients, many of whom struggle to swallow large tablets and manage complex, multi-pill regimens. XTANDI® generated $5.3 billion in 2024; partnerships are now being finalised to enable the global launch of this product.
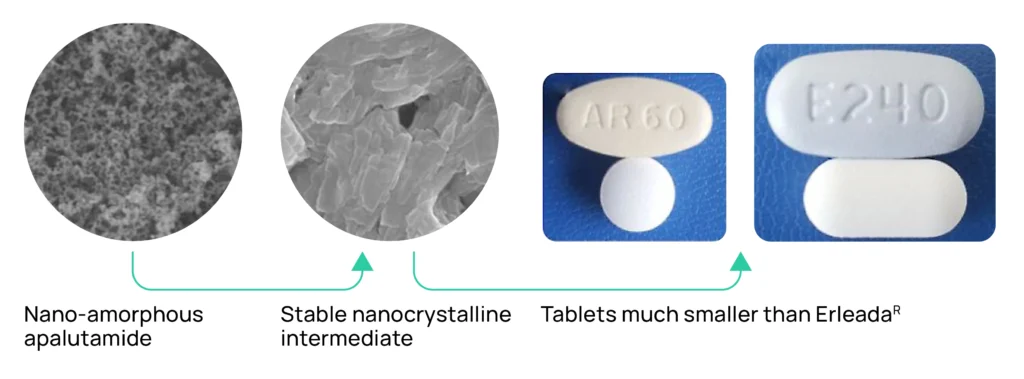
Our development portfolio also includes Nanoapalutamide and Nanoencorafenib, patient-centric versions of Johnson & Johnson’s ERLEADA® (apalutamide (prostate cancer)) and Pfizer’s BRAFTOVI ® (encorafenib (lung, skin and colorectal cancers)). These innovations are also designed to reduce tablet burden and improve regimen compliance. ERLEADA® sold $3.0B in 2024 and BRAFTOVI®/MEKTOVI® sold together totalled $607M.
Beyond these near-term launches, Nanoform’s pipeline includes multiple additional programmes at various stages of development, as can be seen above, all designed to “NanoImprove” important medicines. We welcome enquiries from potential partners and stakeholders who would like to learn more about our technology or discuss co-development or licensing opportunities. For further information, please contact us at info@nanoform.com.